Translate this page into:
Effects of Venusia Max Lotion on skin hydration and skin barrier in patients with dry skin

*Corresponding author: Monil Yogesh Neena Gala, Department of Medical Affairs, Dr. Reddy’s Laboratories Ltd., Hyderabad, Telangana, India. monil.yogesh@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Gala MY, Muchhala SS, Charugulla SN, Rathod R, Mane A, Pandit S, et al. Effects of Venusia Max Lotion on skin hydration and skin barrier in patients with dry skin. CosmoDerma 2021;1:41.
Abstract
Objectives:
The objectives of the study were to evaluate skin hydration and transepidermal water loss (TEWL), after the application of the test product (Venusia Max Lotion [Paraben, Alcohol, Mineral Oil, and Animal Origin (PAMA)] free).
Material and Methods:
The study was a single-center, non-randomized, observational study. Test product was compared to control sites after application on volar forearms of women with dry skin. Hydration and TEWL measurements at baseline, after 12 hours, 24 hours and 36 hours of product application were done under the occluded and unoccluded condition.
Results:
The study was completed with 30 female subjects. Increase in the mean MMSC values was significantly greater on test product site as compared to control site, at all-time points. For TEWL readings over 36 h, when kept occluded and unoccluded, respectively, there were no significant differences in TEWL readings between the test product site and control site at any time points.
Conclusion:
The test product, Venusia Max Lotion (PAMA free), can be useful in maintaining the skin barrier properties and significantly improving skin hydration in individuals with dry skin or dry skin-related conditions.
Keywords
Moisturizer
Skin hydration
Transepidermal water loss
Dry skin
INTRODUCTION
Skin performs numerous functions and is the most effective barrier between the external environment and the internal physiological environment. Functioning of the stratum corneum (SC) is essential for healthy skin.[1] SC helps to keep water in or on the skin by its number of natural systems with water-repellent lamellar bilayers. SC helps to restrict the outward flow of water and electrolytes and prohibits the absorption of hazardous substances and microbial pathogens.[2] Dry skin (xeroderma) is a commonly encountered clinical diagnosis in dermatological clinics. It is crucial to maintain skin moisture to prevent dry skin. Moisturizers help to improve and maintain the skin barrier function and thereby prevent dry skin. Moisturizer acts to prevent and reduce transepidermal water loss (TEWL) from the skin.[3]
Moisturizers are integral to maintain healthy skin in day-to-day life. A study from the United States observed that among the over-the-counter skin products, moisturizers were the third most commonly utilized skin products after the hydrocortisone and anti-infective agents.[4]
Therefore, assessment of moisturizers for their moisturizing effect and effect on skin barrier function is essential. Multiple methods varying from traditional ones such as probe-based devices to spectroscopic and imaging modalities and skin capacitance methods are used to assess skin hydration effects. Skin capacitance is considered a gold standard method for determining the moisturizing effect.[5] In addition, skin barrier function assessment is essential as loss of integrity of SC can lead to increased TEWL. Using closed chamber methods, more accurate and rapid readings can be obtained.[5] Given the moisturizers being an important part of a dermatologist’s armamentarium,[4] we assessed our moisturizer – Venusia Max Lotion (PAMA free) for its moisturizing effects in healthy females with dry skin.
MATERIAL AND METHODS
Setting and design
This single-center, observational study was conducted at a C.L.A.I.M.S. Pvt. Ltd. in Mumbai, India, by a qualified investigator. The study was approved by Independent Ethics Committee, C.L.A.I.M.S. Pvt. Ltd., Andheri (W), Mumbai. The study was conducted respecting the principle of the Declaration of Helsinki and its amendments in conformity with the Good Clinical Practices principles and Schedule Y. The study, including potential risks and benefits, was explained to the participants by the Principal Investigator/Coinvestigator before screening for the study. All volunteer queries were cleared by the Principal Investigator/Coinvestigator and willing participants consented for the study.
Participants
In this study, women aged between 18 and 55 years having dry skin type (MoistureMeter SC [MMSC] [Delfin Technologies Ltd., Finland] readings <20) who had healthy skin on the forearms without any scars moles or tattoos and who consented for the participants were included. Females who were menopausal, pregnant, or lactating, had hypersensitivity to any ingredient of the test product, those who were taking medical treatment (systemic or topical) in the past 1 month that could interfere with study results, females with a chronic illness that may interfere with skin sensitivity and study results (such as atopic dermatitis, psoriasis, eczema, hypothyroidism, hormonal disorders, etc.), and females who were part of other clinical investigation till the past 1 month were excluded from the study.
Procedures
After reading, understanding, and signing the informed consent document, participants were recruited for the study based on the inclusion/exclusion criteria. The forearms of each participant were cleaned, and the participants were acclimatized under controlled conditions for a duration of about 30 min at the beginning of the study. The humidity was maintained between 40 and 60% and temperature at 20–22°C for the entire study visit duration. In total eight sites (2 × 2 cm2), four for test product and four for control sites (without product application) were marked on volar forearms of participants. Baseline MMSC and VapoMeter (VM) (Delfin Technologies Ltd., Finland) measurements were carried out on eight test sites.
Approximately 0.030 g of the test product (Venusia Max Lotion [PAMA free]) containing shea butter, mango butter, cocoa butter, aloe butter, cetyl alcohol, stearic acid, emulsifying waxes, cyclomethicone, dimethicone, phenoxyethanol, propylene glycol, glycerin, disodium EDTA, zinc oxide, and purified water; batch no.: VEN-1-F-015) was applied on the four test sites (sites 1, 3, 5, and 7) by massaging for 30 s using a fingerstall. There was no product application at the four control sites (sites 2, 4, 6, and 8). Sites 1, 2, 3, 4, 5, and 6 were occluded whereas sites 7 and 8 remained unoccluded for the entire study duration [Figure 1].
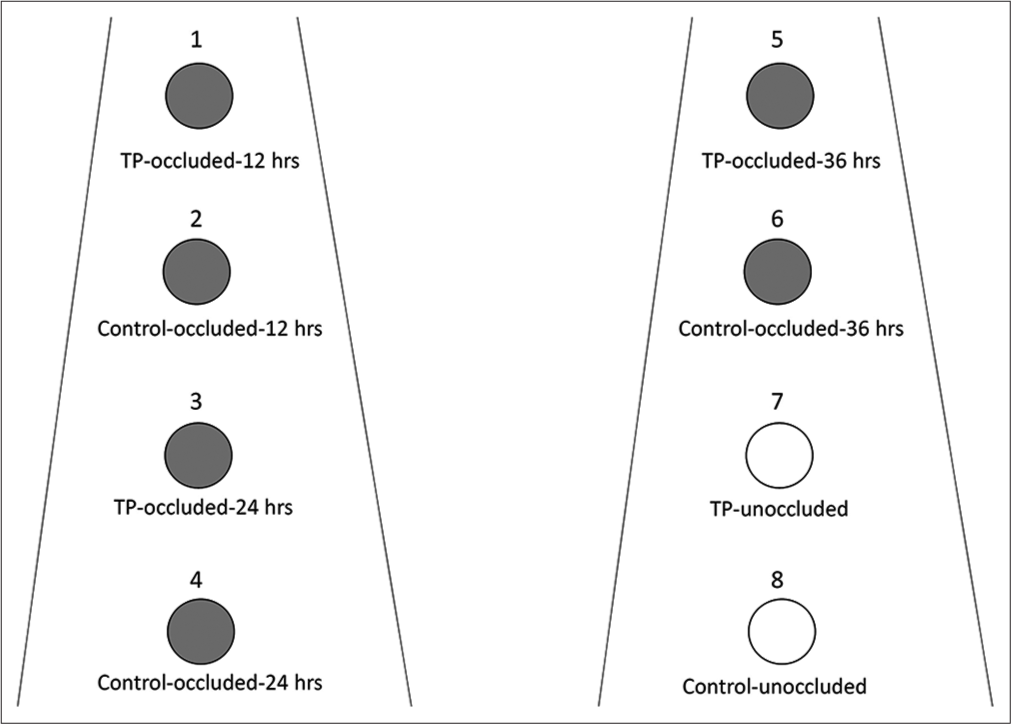
- Sites of application of test product and control sites. TP: Test product; gray circles: Indicated occluded sites for the specified duration, open circles: Unoccluded sites.
At 12 h, occlusion on sites 1 (test) and 2 (control) was removed. Similarly, at 24 h and 36 h, occlusion on sites 3 (test) and 4 (control) as well as at sites 5 (test) and 6 (control) was removed. MMSC and VM measurements were done after each time period from the sites where occlusion was removed as well as of the sites which were left unoccluded (sites 7 and 8). Qualified study personnel carried out the procedures. The observations were entered in the case record pro forma.
Outcomes measures
The primary outcomes assessed in the study were measurement of skin hydration of SC (using MMSC) and effect on skin barrier function (using VM).
The MMSC measures the hydration of the skin surface, that is, SC. The electrical properties of the skin layers are related to their water content. The probe, the skin surface, and the deeper skin layers form a structure, similar to an electrical capacitor. The measured capacitance is proportional to the water content of the surface layer of the skin. The higher the reading, the higher the moisture content. MMSC readings of <20, 20–40, and >40 indicate dry skin, normal skin, and well-hydrated skin, respectively.
The VM is equipped with a closed cylindrical chamber that contains sensors for relative humidity and temperature. There is a linear increase of relative humidity (RH%) in the chamber shortly after placing the device in contact with the skin. The TEWL is calculated from the increase in RH%. Values of ambient RH (%) and temperature (°C) are recorded before skin contact. The chamber is passively ventilated between measurements. Reduction in the VM readings indicates a significant reduction in moisture loss from the skin.
Statistical analysis
Statistical analysis was carried out by an independent statistician using version 10.0 of statistical software SPSS. Continuous variables were summarized by the treatment group using summary statistics (number of observations, mean, standard deviation, or median with a range of minimum and maximum). Mean differences of continuous variables such as MMSC readings and VM readings were estimated by Student’s t-test and ANOVA (with repeated measures) with postdoc by Bonferroni. P < 0.05 was considered significant for statistical comparisons.
RESULTS
Baseline characteristics
The study period was from November 18, 2020, to December 10, 2020. There were 30 female participants included in the study. The mean age was 28.57 ± 9.47 years and ranged from 19 years to 46 years. There were no dropouts in the study.
Skin hydration changes in occluded suites
Table 1 shows the changes in values on MMSC. At baseline, sites of application of test product and control sites had no significant difference in skin hydration as indicated by MMSC values. The MMSC value at each site of test and control was <20 indicating dry skin.
| Parameters | Observations (n=30) |
|---|---|
| Age (years) | |
| Mean±SD | 28.57±9.47 |
| Range | 19–46 |
| Female sex | 30 (100.0) |
At 12 h, a significant increase in skin hydration was seen in the test site compared to the control site (mean difference in MMSC value: 28.55 ± 13.22 vs. 5.87 ± 4.56; P = 0.0001). Similarly, at 24 h (mean difference in MMSC value: 19.16 ± 12.51 vs. 2.85 ± 5.04; P = 0.0001) and 36 h (mean difference in MMSC value: 18.67 ± 11.04 vs. 4.96 ± 4.66;P = 0.001), MMSC readings were significantly higher at test sites than control sites [Table 2]. Changes in MMSC values in two groups are schematically represented in Figure 2.
| Sites | Time period | MMSC readings | P-value | |
|---|---|---|---|---|
| Venusia Max Lotion (PAMA free) | Control site | |||
| Site 1 (test) and 2 (control) | Baseline | 12.05±02.45 | 12.22±2.70 | 0.8000 |
| At 12 h | 40.60±13.54 | 18.09±6.08 | - | |
| Mean difference | 28.55±13.22 | 5.87±4.56 | 0.0001 | |
| Site 3 (test) and 4 (control) | Baseline | 12.77±2.75 | 13.25±2.81 | 0.506 |
| At 24 h | 31.93±13.09 | 16.10±5.66 | - | |
| Mean difference | 19.16±12.51 | 2.85±5.04 | 0.0001 | |
| Site 5 (test) and 6 (control) | Baseline | 18.85±3.01 | 12.59±2.63 | 0.315 |
| At 36 h | 30.52±11.11 | 17.54±4.92 | - | |
| Mean difference | 18.67±11.04 | 4.96±4.66 | 0.001 | |

- Changes in mean MMSC readings in two groups at different time periods. (a) At 12 h. (b) At 24 h. (c) At 36 h.
Skin hydration changes in unoccluded suites
An increase in mean MMSC values was significantly more on the test product site as compared to the control site, at all-time points [Table 3].
| Time periods | MMSC readings | P-value | |
|---|---|---|---|
| Venusia Max Lotion (PAMA free) | Control site | ||
| Baseline | 12.97±2.80 | 13.29±2.92 | 0.666 (NS) |
| 12 h | 15.73±3.25 | 14.29±2.77 | - |
| 24 h | 18.24±4.22 | 14.89±3.00 | - |
| 36 h | 15.27±2.74 | 14.35±2.89 | - |
| Mean difference (Baseline – 12 h) | 2.76±2.71 | 0.99±1.36 | 0.002 |
| Mean difference (Baseline – 24 h) | 5.27±3.76 | 1.60±1.76 | 0.001 |
| Mean difference (Baseline – 36 h) | 2.30±2.33 | 1.06±1.50 | 0.023 |
Measurement of TEWL by VM
Table 4 shows the VM reading at different time periods in test and control sites under occluded conditions. There was no significant difference in the baseline readings at each site at each time period. The mean difference for change in VM readings in test and control sites did not show a significant difference at 12 h (P = 0.308), 24 h (P = 0.632), and 36 h (P = 0.237).
| Sites | Time period | Venusia Max Lotion (PAMA free) | Control site | P-value |
|---|---|---|---|---|
| Site 1 (test) and 2 (control) | Baseline | 9.29±1.89 | 9.55±2.66 | 0.644 |
| At 12 h | 8.56±2.68 | 9.49±2.67 | - | |
| Mean difference | –0.73±2.50 | –0.06±2.55 | 0.308 | |
| Site 3 (test) and 4 (control) | Baseline | 10.04±2.27 | 9.86±2.24 | 0.758 |
| At 24 h | 11.19±2.08 | 11.28±2.12 | - | |
| Mean difference | 1.15±2.06 | 1.42±2.28 | 0.632 | |
| Site 5 (test) and 6 (control) | Baseline | 9.51±2.51 | 9.67±2.39 | 0.801 |
| At 36 h | 9.48±2.57 | 10.23±2.51 | - | |
| Mean difference | –0.03±2.13 | 0.56±1.67 | 0.237 |
Similarly, under unoccluded conditions, there were no significant differences in VM readings at 12 h (P = 0.730), 24 h (P = 0.990), and 36 h (P = 0.440) between the test and control sites, as depicted in Table 5.
| Time periods | Venusia Max Lotion (PAMA free) | Control site | P-value |
|---|---|---|---|
| Baseline | 8.98±2.36 | 9.76±2.39 | 0.208 |
| 12 h | 8.65±1.97 | 9.62±2.15 | - |
| 24 h | 9.52±2.24 | 10.28±1.92 | - |
| 36 h | 9.73±2.24 | 10.16±2.21 | - |
| Mean difference (Baseline – 12 h) | –0.33±2.57 | –0.14±1.20 | 0.730 |
| Mean difference (Baseline – 24 h) | 0.54±3.19 | 0.52±1.81 | 0.990 |
| Mean difference (Baseline – 36 h) | 0.75±2.18 | 0.40±1.18 | 0.440 |
Safety assessments
None of the participants reported any skin reactions or intolerances after the test product application. There were no adverse events reported during the entire study duration by any participant.
DISCUSSION
The term “Moisturizer” clearly denotes the moisturizing effect, but additional ingredients exert anti-inflammatory, anti-pruritic, anti-mitotic, anti-microbial, and photoprotective effects.[3] They also significantly improve quality of life.[6] Dry skin is among the most commonly encountered conditions in dermatology consultations and rates may be as high as 92.5% of patients visiting a skin specialist.[7] In combating dry skin, one needs to ensure that the skin is effectively hydrated through the use of humectants and improve the skin barrier function. With the use of moisturizers, there is an increase in water content in the SC that helps in keeping the corneocytes together. Furthermore, they strengthen the weakened skin barrier function.[3] In assessing the moisturizing effect, we observed that moisturizer Venusia Max Lotion (PAMA free) showed significantly higher skin hydration effects at 12, 24, and 36 h after application compared to no application of moisturizer. Thus, individuals with dry skin can get an excellent moisturizing effect from Venusia Max Lotion (PAMA free) application. The effect was measured using the MMSC device that assesses the moisturizing effect with a gold standard capacitance method.[5] In evaluating the effects of moisturizers, Spada et al. observed that ceramide cream and three other reference moisturizers showed a significant increase in skin hydration overtime after a single application.[8] In another study, Constantin et al. assessed the skin hydration effect of moisturizers in patients of allergic contact dermatitis (ACD) and healthy individuals. They observed a statistically significant increase in skin hydration after applying the moisturizer. In both groups, patients with ACD and healthy subjects, the effect of hydration lasted for 28 days. In healthy subjects, the increase in hydration was lower but progressive but remained significant compared to patients with ACD.[9] Both these studies used Corneometer for assessing skin hydration which also employs the same principle of capacitance method. These findings indicate that moisturizers help in improving skin hydration significantly over short term and may maintain the effect for some longer time. In addition to these, multiple other studies demonstrated that moisturizers are effective in improving skin hydration.[10-12] These results are consistent with our findings indicating good efficacy of our moisturizer in improving skin hydration in healthy subjects with dry skin.
Another important consideration with the use of moisturizers is skin barrier function. It is crucial to understand the effects of different ingredients on skin barrier function. Some ingredients such as emulsifiers may weaken the skin barrier while petrolatum provides an immediate barrier repairing effect.[13] We observed no significant differences in TEWL as measured by VM with the use of moisturizer compared to control (i.e., no use of moisturizer). It indicates skin barrier function of healthy women with dry skin was preserved till 36 h after applications. Some studies established that moisturizers also improve skin barrier function by reducing TEWL.[12,14] The compositional differences in moisturizers can have different effects on TEWL. A study by Crowther et al. reported that from the three commercially available moisturizers, only one showed the increase in SC thickness as indicated by increase epidermal lipogenesis and SC barrier function. It was possibly because of the compositional differences between the products and was attributable to the presence of niacinamide in one of the tested moisturizers.[10] The findings from Samadi et al. support such observation. They noted that the TEWL significantly decreased with the use of two moisturizers. However, termination of moisturizers after 2 weeks of application, only one showed significant persistence of effect even after 1 week of discontinuation compared to the control site.[15] It is, therefore, emphasized that maintaining and/or improving skin barrier function is essential to preserve proper skin functioning. In addition, being free from parabens and alcohol, this lotion has lower risk of irritancy and skin sensitivity. Furthermore, being free of mineral oil, the risk of trapping other pore-clogging ingredients in the skin pores is non-existent.
CONCLUSION
Moisturizers are the cornerstone of dry skin and related dermatological disorders and are one of the cosmetic products used daily. We identify that the moisturizer product Venusia Max Lotion (PAMA free) evaluated in this study significantly improves skin hydration and maintains skin barrier function without any untoward side effects. Thus, moisturizer Venusia Max Lotion (PAMA free) can be for useful in maintaining hydration and skin barrier function in individuals with dry skin and related conditions.
Acknowledgement
We thank Dr. Vijay M. Katekhaye (Quest MedPharma Consultants, Nagpur) for his assistance in drafting, editing and reviewing the manuscript.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Dr. Reddy’s Laboratories Ltd., Hyderabad, India.
Conflicts of interest
Dr. Monil Yogesh Neena Gala, Snehal Sameer Muchhala, Dr. Sujeet Narayan Charugulla, Dr. Rahul Rathod, Dr. Amey Mane, Sucheta Pandit, Alok Ranjan Samal, and Anup Avijit Choudhury are salaried employees of Dr. Reddy’s Laboratory Ltd., Hyderabad, India.
References
- The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
- [Google Scholar]
- A look at epidermal barrier function in atopic dermatitis: Physiologic lipid replacement and the role of ceramides. Skin Therapy Lett. 2012;17:6-9.
- [Google Scholar]
- Moisturizers: The slippery road. Indian J Dermatol. 2016;61:279-87.
- [CrossRef] [PubMed] [Google Scholar]
- Over-the-counter topical skin products-a common component of skin disease management. Cutis. 2004;74:55-67.
- [Google Scholar]
- Review of modern techniques for the assessment of skin hydration. Cosmetics. 2019;6:19.
- [CrossRef] [Google Scholar]
- Wet skin moisturizer: A novel approach for xerotic skin: 5590. J Am Acad Dermatol. 2017;76:AB282.
- [CrossRef] [Google Scholar]
- Epidemiological pattern of psoriasis, vitiligo and atopic dermatitis in India: Hospital-based point prevalence. Indian Dermatol Online J. 2014;5(Suppl 1):S6-8.
- [CrossRef] [PubMed] [Google Scholar]
- Skin hydration is significantly increased by a cream formulated to mimic the skin's own natural moisturizing systems. Clin Cosmet Investig Dermatol. 2018;11:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Skin hydration assessment through modern non-invasive bioengineering technologies. Maedica (Bucur). 2014;9:33-8.
- [Google Scholar]
- Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br J Dermatol. 2008;159:567-77.
- [CrossRef] [PubMed] [Google Scholar]
- Targeted dry skin treatment using a multifunctional topical moisturizer. Int J Cosmet Sci. 2021;43:191-200.
- [CrossRef] [PubMed] [Google Scholar]
- Combined effects of glycerol and petrolatum in an emollient cream: A randomized, double-blind, crossover study in healthy volunteers with dry skin. J Cosmet Dermatol. 2020;19:1399-403.
- [CrossRef] [PubMed] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [Google Scholar]
- A novel moisturizer with high sun protection factor improves cutaneous barrier function and the visible appearance of rosacea-prone skin. J Cosmet Dermatol. 2019;18:1686-92.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term effects of two 24-hour moisturizing products on skin barrier structure and function: A biometric and molecular study. Health Sci Rep. 2021;4:e308.
- [CrossRef] [Google Scholar]






